Niobium (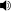 /naɪˈoʊbiəm/) or columbium (/kəˈlʌmbiəm/), is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite. The name comes from Greek mythology: Niobe, daughter ofTantalus.
/naɪˈoʊbiəm/) or columbium (/kəˈlʌmbiəm/), is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite. The name comes from Greek mythology: Niobe, daughter ofTantalus.
Niobium has physical and chemical properties similar to those of the element tantalum, and the two are therefore difficult to distinguish. The English chemist Charles Hatchett reported a new element similar to tantalum in 1801, and named it columbium. In 1809, the English chemist William Hyde Wollaston wrongly concluded that tantalum and columbium were identical. The German chemist Heinrich Rose determined in 1846 that tantalum ores contain a second element, which he named niobium. In 1864 and 1865, a series of scientific findings clarified that niobium and columbium were the same element (as distinguished from tantalum), and for a century both names were used interchangeably. The name of the element was officially adopted as niobium in 1949.
It was not until the early 20th century that niobium was first used commercially. Brazil is the leading producer of niobium and ferroniobium, an alloy of niobium and iron. Niobium is used mostly in alloys, the largest part in special steel such as that used in gas pipelines. Although alloys contain only a maximum of 0.1%, that small percentage of niobium improves the strength of the steel. The temperature stability of niobium-containing superalloys is important for its use in jet and rocket engines. Niobium is used in various superconducting materials. These superconducting alloys, also containing titanium and tin, are widely used in thesuperconducting magnets of MRI scanners. Other applications of niobium include its use in welding, nuclear industries, electronics, optics, numismatics and jewelry. In the last two applications, niobium's low toxicity and ability to be colored by anodization are particular advantages.


History
Niobium was discovered by the English chemist Charles Hatchett in 1801.[1] He found a new element in a mineral sample that had been sent to England from Massachusetts, United States in 1734 by a John Winthrop,[2] and named the mineral columbite and the new elementcolumbium after Columbia, the poetical name for America.[3] The columbium discovered by Hatchett was probably a mixture of the new element with tantalum.[3]
Subsequently, there was considerable confusion[4] over the difference between columbium (niobium) and the closely related tantalum. In 1809, the English chemist William Hyde Wollaston compared the oxides derived from both columbium—columbite, with a density 5.918 g/cm3, and tantalum—tantalite, with a density over 8 g/cm3, and concluded that the two oxides, despite the significant difference in density, were identical; thus he kept the name tantalum.[4] This conclusion was disputed in 1846 by the German chemist Heinrich Rose, who argued that there were two different elements in the tantalite sample, and named them after children of Tantalus: niobium (from Niobe), andpelopium (from Pelops).[5][6] This confusion arose from the minimal observed differences between tantalum and niobium. The claimed new elements pelopium, ilmenium and dianium[7] were in fact identical to niobium or mixtures of niobium and tantalum.[8]
The differences between tantalum and niobium were unequivocally demonstrated in 1864 by Christian Wilhelm Blomstrand,[8] and Henri Etienne Sainte-Claire Deville, as well as Louis J. Troost, who determined the formulas of some of the compounds in 1865[8][9] and finally by the Swiss chemist Jean Charles Galissard de Marignac[10] in 1866, who all proved that there were only two elements. Articles on ilmeniumcontinued to appear until 1871.[11]
De Marignac was the first to prepare the metal in 1864, when he reduced niobium chloride by heating it in an atmosphere of hydrogen.[12]Although de Marignac was able to produce tantalum-free niobium on a larger scale by 1866, it was not until the early 20th century that niobium was first used commercially, in incandescent lamp filaments.[9] This use quickly became obsolete through the replacement of niobium with tungsten, which has a higher melting point and thus is preferable for use in incandescent lamps. The discovery that niobium improves the strength of steel was made in the 1920s, and this application remains its predominant use.[9] In 1961 the American physicistEugene Kunzler and coworkers at Bell Labs discovered that niobium-tin continues to exhibit superconductivity in the presence of strong electric currents and magnetic fields,[13] making it the first material to support the high currents and fields necessary for useful high-power magnets and electrically powered machinery. This discovery would allow — two decades later — the production of long multi-strand cables that could be wound into coils to create large, powerful electromagnets for rotating machinery, particle accelerators, or particle detectors.[14][15]
Naming of the element
Columbium (symbol Cb[16]) was the name originally given to this element by Hatchett, and this name remained in use in American journals—the last paper published by American Chemical Society with columbium in its title dates from 1953[17]—while niobium was used in Europe. To end this confusion, the name niobium was chosen for element 41 at the 15th Conference of the Union of Chemistry in Amsterdam in 1949.[18] A year later this name was officially adopted by the International Union of Pure and Applied Chemistry (IUPAC) after 100 years of controversy, despite the chronological precedence of the name Columbium.[18] The latter name is still sometimes used in US industry.[19] This was a compromise of sorts;[18] the IUPAC accepted tungsten instead of wolfram, in deference to North American usage; and niobium instead of columbium, in deference to European usage. Not everyone agreed, and while many leading chemical societies and government organizations refer to it by the official IUPAC name, many leading metallurgists, metal societies, and the United States Geological Survey still refer to the metal by the original "columbium".[20][21]
Characteristics
Physical
Niobium is a lustrous, grey, ductile, paramagnetic metal in group 5 of the periodic table (see table), although it has an atypical configuration in its outermost electron shells compared to the rest of the members. (This can be observed in the neighborhood of ruthenium (44), rhodium (45), and palladium (46).)
| Z | Element | No. of electrons/shell |
|---|---|---|
| 23 | vanadium | 2, 8, 11, 2 |
| 41 | niobium | 2, 8, 18, 12, 1 |
| 73 | tantalum | 2, 8, 18, 32, 11, 2 |
| 105 | dubnium | 2, 8, 18, 32, 32, 11, 2 |
Niobium becomes a superconductor at cryogenic temperatures. At atmospheric pressure, it has the highest critical temperature of the elemental superconductors: 9.2 K.[22] Niobium has the largest magnetic penetration depth of any element.[22] In addition, it is one of the three elemental Type II superconductors, along with vanadium and technetium. The superconductive properties are strongly dependent on the purity of the niobium metal.[23] When very pure, it is comparatively soft and ductile, but impurities make it harder.[24]
The metal has a low capture cross-section for thermal neutrons;[25] thus it is used in the nuclear industries.[26]
Chemical
The metal takes on a bluish tinge when exposed to air at room temperature for extended periods.[27] Despite presenting a high melting point in elemental form (2,468 °C), it has a low density in comparison to other refractory metals. Furthermore, it is corrosion resistant, exhibits superconductivity properties, and formsdielectric oxide layers.
Niobium is slightly less electropositive and more compact than its predecessor in the periodic table, zirconium, whereas it is virtually identical in size to the heavier tantalum atoms, owing to the lanthanide contraction.[24] As a result, niobium's chemical properties are very similar to those for tantalum, which appears directly below niobium in the periodic table.[9] Although its corrosion resistance is not as outstanding as that of tantalum, its lower price and greater availability make niobium attractive for less demanding uses such as linings in chemical plants.[24]
Isotopes
Main article: Isotopes of niobium
Naturally occurring niobium is composed of one stable isotope, 93Nb.[28] As of 2003, at least 32 radioisotopes have also been synthesized, ranging in atomic mass from 81 to 113. The most stable of these is 92Nb with ahalf-life of 34.7 million years. One of the least stable is 113Nb, with an estimated half-life of 30 milliseconds. Isotopes that are lighter than the stable 93Nb tend to decay by β+ decay, and those that are heavier tend to decay by β- decay, with some exceptions. 81Nb, 82Nb, and 84Nb have minor β+ delayed proton emission decay paths, 91Nb decays by electron capture and positron emission, and 92Nb decays by both β+ and β-decay.[28]
At least 25 nuclear isomers have been described, ranging in atomic mass from 84 to 104. Within this range, only 96Nb, 101Nb, and 103Nb do not have isomers. The most stable of niobium's isomers is 93mNb with a half-life of 16.13 years. The least stable isomer is 84mNb with a half-life of 103 ns. All of niobium's isomers decay by isomeric transition or beta decay except 92m1Nb, which has a minor electron capture decay chain.[28]
Occurrence
See also: Category:Niobium minerals
Niobium is estimated to be 33rd on the list of the most common elements in the Earth’s crust with 20 ppm.[29] Some think that the abundance on Earth should be much greater, but that the “missing” niobium may be located in the Earth’s core due to the metal's high density.[20] The free element is not found in nature, but it does occur in minerals.[24] Minerals that contain niobium often also contain tantalum, such as columbite((Fe,Mn)(Nb,Ta)2O6) and columbite-tantalite (or coltan, (Fe,Mn)(Ta,Nb)2O6).[30] Columbite-tantalite minerals are most usually found as accessory minerals in pegmatite intrusions, and in alkaline intrusive rocks. Less common are the niobates of calcium, uranium, thorium and the rare earth elements such as pyrochlore ((Na,Ca)2Nb2O6(OH,F)) and euxenite ((Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6). These large deposits of niobium have been found associated with carbonatites (carbonate-silicate igneous rocks) and as a constituent of pyrochlore.[31]
The two largest deposits of pyrochlore were found in the 1950s in Brazil and Canada, and both countries are still the major producers of niobium mineral concentrates.[9] The largest deposit is hosted within a carbonatite intrusion at Araxá, Minas Gerais Brazil, owned by CBMM (Companhia Brasileira de Metalurgia e Mineração); the other deposit is located at Goiás owned by Anglo American plc (through its subsidiary Mineração Catalão), also hosted within a carbonatite intrusion.[32] Altogether these two Brazilian mines produce around 75% of world supply. The third largest producer of niobium is the carbonatite-hosted Niobec Mine, Saint-Honoré near Chicoutimi, Quebec owned by Iamgold Corporation Ltd, which produces around 7% of world supply.[32]
Extensive unexploited resources are located in Nigeria, Democratic Republic of Congo, Tanzania, Malawi, Australia, Afghanistan, Russia and Colombia.

No comments:
Post a Comment