The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.[1][2][3] Discovered independently by Charles-Adolphe Wurtz[4][5][6] and Alexander Porfyrevich Borodin in 1872,[7] the reaction combines two carbonyl compounds (the original experiments used aldehydes) to form a new β-hydroxy carbonyl compound. These products are known as aldols, from the aldehyde + alcohol, a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic.[8][9][10] For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritol[11] and the synthesis of the heart disease drug Lipitor (atorvastatin).[12][13]
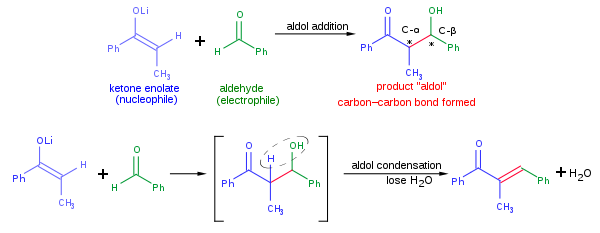
The aldol reaction is powerful because it unites two relatively simple molecules into a more complex one. Increased complexity arises because up to two new stereogenic centers (on the α- and β-carbon of the aldol adduct, marked with asterisks in the scheme below) are formed. Modern methodology is capable of not only allowing aldol reactions to proceed in high yield but also controlling both the relative and absolute stereochemical configuration of these stereocenters. This ability to selectively synthesize a particular stereoisomer is significant because different stereoisomers can have very different chemical or biological properties.
For example, stereogenic aldol units are especially common in polyketides, a class of molecules found in biological organisms. In nature, polyketides are synthesized by enzymes that effect iterative Claisen condensations. The 1,3-dicarbonyl products of these reactions can then be variously derivatized to produce a wide variety of interesting structures. Often, such derivitization involves the reduction of one of the carbonyl groups, producing the aldol subunit. Some of these structures have potent biological properties: the potent immunosuppressant FK506, the anti-tumor agent discodermolide, or the antifungal agentamphotericin B, for example. Although the synthesis of many such compounds was once considered nearly impossible, aldol methodology has allowed their efficient synthesis in many cases.[14]
A typical modern aldol addition reaction, shown above, might involve the nucleophilic addition of a ketone enolate to an aldehyde. Once formed, the aldol product can sometimes lose a molecule of water to form an α,β-unsaturated carbonyl compound. This is called aldol condensation. A variety of nucleophiles may be employed in the aldol reaction, including the enols, enolates, and enol ethers of ketones, aldehydes, and many other carbonyl compounds. The electrophilic partner is usually an aldehyde or ketone (many variations, such as the Mannich reaction, exist). When the nucleophile and electrophile are different, the reaction is called acrossed aldol reaction; on the converse, when the nucleophile and electrophile are the same, the reaction is called aldol dimerization.
No comments:
Post a Comment